
We have talked a lot about clinical studies throughout our website but below actually shows you the results of our studies, as well as some results from a recent consumer marketing survey which we carried out.
Short term safety clinical study using Pureis CBD:
The purpose of this study was to determine suitable doses for a subsequent longer study as detailed below.
Longer term safety clinical study using Pureis CBD:
This longer-term study provides information on the possible health hazards likely to arise from repeated exposure over a prolonged period of time.
During this study there was no sign of adverse behaviour. Various parameters were assessed:
- Clinical observations
- Mobility
- Reaction sensation
- Body temperature
- Ophthalmology (conditions relating to the eye)
- Haematology (blood analysis)
- Clinical chemistry (analysis of body fluids)
- Organ and tissue examination
- Histology (microscopic structure of tissues)
- Sperm evaluation

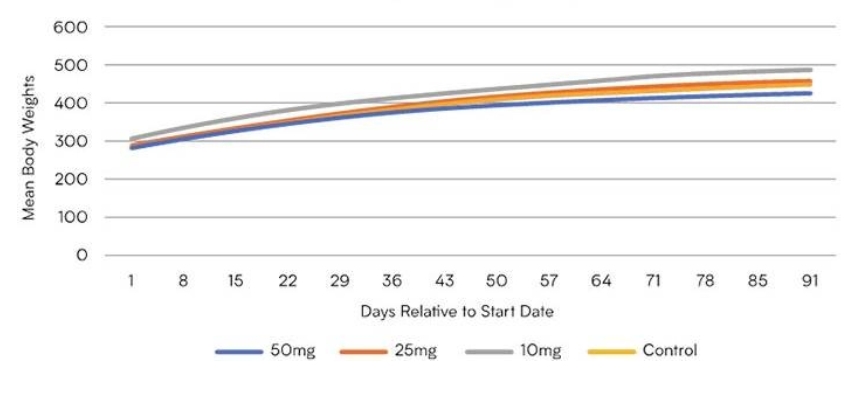
Summary of Body Weights
The control line represents the subjects that did not take CBD during the trail. The body weight of the subjects that took Pureis 10mg, 25mg and 50mg of CBD had the same body weight as those that did not take CBD. This shows that Pureis CBD was well tolerated and caused no toxic affect to any part of the body.
Conclusion of Pureis CBD studies
The results of our short term and long-term clinical studies demonstrated that our Pureis CBD is well tolerated for consumer use. In addition, we conducted a series of tests to demonstrate that Pureis CBD does not have any mutagenic effects – the results confirmed that Pureis CBD has no evidence of mutagenicity.